
Preparation of dry hydrogen?
klugesmith, Tue Mar 27 2018, 05:45AMA helium-filled mylar balloon purchased last December remained buoyant in air for months.
Now I want to re-fill it with hydrogen, and see how long the balloon can hold that gas.
The amount needed is 10 or 15 liters. On the order of 1/2 mole.
In my high school years, we filled latex balloons with hydrogen by putting aluminum foil in a solution of lye. Usually in a large glass Coke bottle, with a 10 cent deposit, because PET bottles for fizzy beverages hadn't been invented.
Now I'm asking on a forum, before generally searching the Internet, about whether anyone prefers a different reaction. People have been filling balloons with hydrogen since 1783.
I also want to try drying the hydrogen, which starts out hot and humid. Maybe bubble it through ice water before some kind of dessicant column. Suggestions are welcome!
Re: Preparation of dry hydrogen?
Sulaiman, Tue Mar 27 2018, 11:03AM
I would just use what is available;
Hydrogen;
. Aluminium cooking foil + drain cleaner sodium hydroxide
. Lantern Battery zinc or iron wool + brick/concrete cleaner muriatic/hydrochloric acid
. electrolysis of water using carbon rods (painfully slow)
Scrubbing;
. pass gas through a tube loosely packed with dehumidifier calcium chloride or oven-dried cotton wool
. bubble through (drain unblocker) conc. sulphuric acid (hazardous)
I'd try BOC or welding suppliers for bottled gas as other methods will be quite slow.
Sulaiman, Tue Mar 27 2018, 11:03AM
I would just use what is available;
Hydrogen;
. Aluminium cooking foil + drain cleaner sodium hydroxide
. Lantern Battery zinc or iron wool + brick/concrete cleaner muriatic/hydrochloric acid
. electrolysis of water using carbon rods (painfully slow)
Scrubbing;
. pass gas through a tube loosely packed with dehumidifier calcium chloride or oven-dried cotton wool
. bubble through (drain unblocker) conc. sulphuric acid (hazardous)
I'd try BOC or welding suppliers for bottled gas as other methods will be quite slow.
Re: Preparation of dry hydrogen?
the_anomaly, Tue Mar 27 2018, 12:10PM
I built a fuel cell in high school and the chemistry teacher suggested zinc + citric acid with a bit of copper sulfate as a catalyst. Worked great for me and was far safer than the HCL + aluminium I had been trying. I don't have any ratios but the reaction speed is adjustable; I had a steady stream of bubbles from a half filled test tube.
the_anomaly, Tue Mar 27 2018, 12:10PM
I built a fuel cell in high school and the chemistry teacher suggested zinc + citric acid with a bit of copper sulfate as a catalyst. Worked great for me and was far safer than the HCL + aluminium I had been trying. I don't have any ratios but the reaction speed is adjustable; I had a steady stream of bubbles from a half filled test tube.
Re: Preparation of dry hydrogen?
Justin, Tue Mar 27 2018, 06:48PM
Couldn't you just use electrolysis of water as a source of hydrogen?
Justin, Tue Mar 27 2018, 06:48PM
Couldn't you just use electrolysis of water as a source of hydrogen?
Re: Preparation of dry hydrogen?
Sulaiman, Tue Mar 27 2018, 08:25PM
Usually because 2H+ + 2e- = H2
so to produce 0.5 mol H2, 1 mol e- is required = 96485 A.s
96485 A.s = 26.8 amp.hour
Hence my (painfully slow) comment above
Sulaiman, Tue Mar 27 2018, 08:25PM
Usually because 2H+ + 2e- = H2
so to produce 0.5 mol H2, 1 mol e- is required = 96485 A.s

96485 A.s = 26.8 amp.hour
Hence my (painfully slow) comment above
Re: Preparation of dry hydrogen?
Conundrum, Fri Mar 30 2018, 08:31AM
yes, a fuel cell would also work.
Interestingly you can now get these quite cheaply and they have other applications.
Conundrum, Fri Mar 30 2018, 08:31AM
yes, a fuel cell would also work.
Interestingly you can now get these quite cheaply and they have other applications.
Re: Preparation of dry hydrogen?
Sulaiman, Fri Mar 30 2018, 11:57AM
but you will still need at least 26.8 A.h
(more actually as the gas will be slightly compressed, and electrolysis is rarely 100% efficient)
If you want to collect oxygen and/or hydrogen separately then I advise the use of a membrane in the cell as back-pressure on one port can force electrolyte out of the other.
I have not tried, but if you do go the electrolysis way, you could consider a HHO generator,
(all of the hydrogen and oxygen collected together)
lighter than air and MUCH more violent explosion if ignited.
CAUTION: in theory, u.v. light (sunlight) can initiate the reaction, so take care.
Sulaiman, Fri Mar 30 2018, 11:57AM
Conundrum wrote ...
yes, a fuel cell would also work.
Interestingly you can now get these quite cheaply and they have other applications.
yes, a fuel cell would also work.
Interestingly you can now get these quite cheaply and they have other applications.
but you will still need at least 26.8 A.h

(more actually as the gas will be slightly compressed, and electrolysis is rarely 100% efficient)
If you want to collect oxygen and/or hydrogen separately then I advise the use of a membrane in the cell as back-pressure on one port can force electrolyte out of the other.
I have not tried, but if you do go the electrolysis way, you could consider a HHO generator,
(all of the hydrogen and oxygen collected together)
lighter than air and MUCH more violent explosion if ignited.
CAUTION: in theory, u.v. light (sunlight) can initiate the reaction, so take care.
Re: Preparation of dry hydrogen?
2Spoons, Sat Mar 31 2018, 09:22PM
I wouldn't call electrolysis 'painfully' slow. I built a 4 cell electrolyser using stainless steel plates, with gas separation, and with 20A running through it the hydrogen flow was enough to sustain a flame on the end of a 20mm tube. Took 3-4 minutes to fill a plastic shopping bag (about 15l I guess) with mixed H2/O2. The bang when we ignited that was ... impressive.
Personally I'd use the Al + NaOH method to fill a balloon. There are plenty of drying agents you can use - silica gel, NaOH prills, CaO, hell probably even a bucket of dry rice would work well enough for this experiment.
2Spoons, Sat Mar 31 2018, 09:22PM
I wouldn't call electrolysis 'painfully' slow. I built a 4 cell electrolyser using stainless steel plates, with gas separation, and with 20A running through it the hydrogen flow was enough to sustain a flame on the end of a 20mm tube. Took 3-4 minutes to fill a plastic shopping bag (about 15l I guess) with mixed H2/O2. The bang when we ignited that was ... impressive.
Personally I'd use the Al + NaOH method to fill a balloon. There are plenty of drying agents you can use - silica gel, NaOH prills, CaO, hell probably even a bucket of dry rice would work well enough for this experiment.
Re: Preparation of dry hydrogen?
klugesmith, Mon Apr 09 2018, 09:08AM
Thanks, 2Spoons. On the Internet we can find work by other amateurs who got serious about electrolytic H2. I like this one -- the anode and cathode plates are separated by wet felt to keep the gases from mixing. H2 is collected in a bellows at atmospheric pressure. When that's full, a refrigeration compressor pumps the H2 into a storage tank.
Industrial hydrogen is almost all made from methane and other hydrocarbons. Mostly for non-fuel applications, since it's harder to store & contains less energy than its fossil fuel precursors.
Today I made about 7 liters of hydrogen, in a practice run, from zinc and acid.

Without much daylight time left, I deferred the chemical calculations. Broke 88 g of die-cast scrap into chunks that would fit into a 20 ounce Gatorade bottle. Made a hole in the cap for tight fit of a Tygon hose. Added 8 fl. oz. (240 ml) of diluted hydrochloric acid previously used for pickling steel. After displacing all the water from a quart-size canning jar, I started filling a much larger jar. Picture above was taken after about 8 minutes, as the bubbling slowed down.
I ended the experiment at 24 minutes, after the bubbling had almost stopped. I guess from depletion of the acid.



Later, weighed the unconsumed zinc.

Next run might be balloon-filling time. Need to contrive a dessicant column. Also figure in advance the amount of metal and acid, and beware of frothing or overheating since the acid volume and/or concentration need to be increased substantially.
klugesmith, Mon Apr 09 2018, 09:08AM
Thanks, 2Spoons. On the Internet we can find work by other amateurs who got serious about electrolytic H2. I like this one -- the anode and cathode plates are separated by wet felt to keep the gases from mixing. H2 is collected in a bellows at atmospheric pressure. When that's full, a refrigeration compressor pumps the H2 into a storage tank.

Industrial hydrogen is almost all made from methane and other hydrocarbons. Mostly for non-fuel applications, since it's harder to store & contains less energy than its fossil fuel precursors.
Today I made about 7 liters of hydrogen, in a practice run, from zinc and acid.

Without much daylight time left, I deferred the chemical calculations. Broke 88 g of die-cast scrap into chunks that would fit into a 20 ounce Gatorade bottle. Made a hole in the cap for tight fit of a Tygon hose. Added 8 fl. oz. (240 ml) of diluted hydrochloric acid previously used for pickling steel. After displacing all the water from a quart-size canning jar, I started filling a much larger jar. Picture above was taken after about 8 minutes, as the bubbling slowed down.
I ended the experiment at 24 minutes, after the bubbling had almost stopped. I guess from depletion of the acid.



Later, weighed the unconsumed zinc.

Next run might be balloon-filling time. Need to contrive a dessicant column. Also figure in advance the amount of metal and acid, and beware of frothing or overheating since the acid volume and/or concentration need to be increased substantially.
Re: Preparation of dry hydrogen?
Sulaiman, Tue May 08 2018, 04:50PM
klugesmith - you are a bad influence !
... due to your evil influence, I now have four 36" latex balloons and 500g drain cleaner - and I've started stockpiling aluminium cans.
I estimate that my 36" diameter balloons will need about 400 litres of hydrogen each,
I estimate about 25 aluminium cans (c14g each) and a 500g tub of drain cleaner sodium hydroxide to be sure of a little excess hydrogen.
I've not decided how to dry and neutralise (sodium hydroxide droplets) the hydrogen yet,
probably just a wad of cotton wool in a tube.
I hope to levitate 60m of stainless steel wire to act as a temporary antenna.
(UK limit for a tethered balloon)
I intend to start when I have enough cans and these arrive
as I do not want to use my nice glassware for hot sodium hydroxide.
P.S. The only carbonated drink that I buy is Ginger Beer and the plastic coating on the inside and outside of these aluminium cans is very good at resisting sodium hydroxide solution... work in progress.
Sulaiman, Tue May 08 2018, 04:50PM
klugesmith - you are a bad influence !
... due to your evil influence, I now have four 36" latex balloons and 500g drain cleaner - and I've started stockpiling aluminium cans.
I estimate that my 36" diameter balloons will need about 400 litres of hydrogen each,
I estimate about 25 aluminium cans (c14g each) and a 500g tub of drain cleaner sodium hydroxide to be sure of a little excess hydrogen.
I've not decided how to dry and neutralise (sodium hydroxide droplets) the hydrogen yet,
probably just a wad of cotton wool in a tube.
I hope to levitate 60m of stainless steel wire to act as a temporary antenna.
(UK limit for a tethered balloon)
I intend to start when I have enough cans and these arrive

as I do not want to use my nice glassware for hot sodium hydroxide.
P.S. The only carbonated drink that I buy is Ginger Beer and the plastic coating on the inside and outside of these aluminium cans is very good at resisting sodium hydroxide solution... work in progress.
Re: Preparation of dry hydrogen?
2Spoons, Tue May 08 2018, 10:29PM
just be aware that the Al-NaOH method can suffer from thermal runaway. To the point where it starts boiling and then you end up with a balloon full of caustic foam.
A colleague had this happen to him when he was a kid - he was doing this on the bonnet of his dad's car. It did not end well.
2Spoons, Tue May 08 2018, 10:29PM
just be aware that the Al-NaOH method can suffer from thermal runaway. To the point where it starts boiling and then you end up with a balloon full of caustic foam.
A colleague had this happen to him when he was a kid - he was doing this on the bonnet of his dad's car. It did not end well.
Re: Preparation of dry hydrogen?
klugesmith, Thu Jun 21 2018, 06:04PM
The time had come to open a factory-sealed bag of desiccant granules. Found pink indicator beads mixed in with the product. Package says its contents were silica gel and cobalt chloride.
Internet says pink indicates humidity and blue indicates dryness. So the bag must have had a pinhole, or been permeable and old.
Blueness was restored by overnight baking at about 115 °C. Second batch was plenty blue after 2 hours this morning, at barely 100 °C.


klugesmith, Thu Jun 21 2018, 06:04PM
The time had come to open a factory-sealed bag of desiccant granules. Found pink indicator beads mixed in with the product. Package says its contents were silica gel and cobalt chloride.
Internet says pink indicates humidity and blue indicates dryness. So the bag must have had a pinhole, or been permeable and old.
Blueness was restored by overnight baking at about 115 °C. Second batch was plenty blue after 2 hours this morning, at barely 100 °C.


Re: Preparation of dry hydrogen?
klugesmith, Sun Jun 24 2018, 04:22AM
Got the balloon re-levitated today, after looking up humidity numbers. Moisture's especially significant if the aqueous reaction is allowed to get hot. Sulaiman, you may wish to spot check my numbers here:
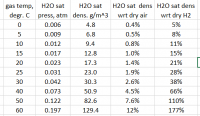
For my estimated balloon volume, plus an allowance for flushing gas and spillage, there would be about 0.5 g of water at 30 degrees C or less than 0.1 g at 0 degrees C.
Reaction vessel and chemicals were same as reported before, and once again immersed in a water bath outdoors.
New plumbing includes a bubbler filled with crushed ice and water, then a DIY column with a huge surplus of silica gel desiccant confined between wads of cotton, then an adapter to a plastic soda straw with which the old balloon had been catheterized.

It took about 1/2 hour from first mixing of chemicals to pinching off the balloon. 200 ml of acid in first batch, drained off and replaced with 250 ml more acid for batch 2. 50 g of die-cast scrap was reduced (not in the chemical sense) to 11 g, a loss of 0.6 mol if it were 100% Zn. Generally consistent with the 1/2 mol of H2 in old estimate. I didn't weigh the empty balloon, but the refilled one has a net buoyancy of about 2 grams.
The desiccant column indicator color changed slightly at the very bottom (inlet side).

Now we'll see how long hydrogen keeps the balloon up.
[edit]Must remember that desiccant has one s and two c's, just like analemma has one n and two m's.
Am thinking of measuring the acid concentrations (fresh and spent) by a more precise titration. Looking for suggestions.
Household bases on hand in liquid form are ammonia and drain opener (not sure how well their concentrations are known).
In solid form there's baking soda and lye (not sure how predictable is their hydration state). I suppose the solids could be baked dry before weighing, as with the desiccant granules the other day.
klugesmith, Sun Jun 24 2018, 04:22AM
Got the balloon re-levitated today, after looking up humidity numbers. Moisture's especially significant if the aqueous reaction is allowed to get hot. Sulaiman, you may wish to spot check my numbers here:

For my estimated balloon volume, plus an allowance for flushing gas and spillage, there would be about 0.5 g of water at 30 degrees C or less than 0.1 g at 0 degrees C.
Reaction vessel and chemicals were same as reported before, and once again immersed in a water bath outdoors.
New plumbing includes a bubbler filled with crushed ice and water, then a DIY column with a huge surplus of silica gel desiccant confined between wads of cotton, then an adapter to a plastic soda straw with which the old balloon had been catheterized.

It took about 1/2 hour from first mixing of chemicals to pinching off the balloon. 200 ml of acid in first batch, drained off and replaced with 250 ml more acid for batch 2. 50 g of die-cast scrap was reduced (not in the chemical sense) to 11 g, a loss of 0.6 mol if it were 100% Zn. Generally consistent with the 1/2 mol of H2 in old estimate. I didn't weigh the empty balloon, but the refilled one has a net buoyancy of about 2 grams.
The desiccant column indicator color changed slightly at the very bottom (inlet side).

Now we'll see how long hydrogen keeps the balloon up.
[edit]Must remember that desiccant has one s and two c's, just like analemma has one n and two m's.
Am thinking of measuring the acid concentrations (fresh and spent) by a more precise titration. Looking for suggestions.
Household bases on hand in liquid form are ammonia and drain opener (not sure how well their concentrations are known).
In solid form there's baking soda and lye (not sure how predictable is their hydration state). I suppose the solids could be baked dry before weighing, as with the desiccant granules the other day.
Re: Preparation of dry hydrogen?
klugesmith, Mon Jun 25 2018, 03:48PM
Bringing back childhood memories, I got some pure zinc metal by taking apart batteries. What was left of it, in cells from the b-waste bin. Anybody need some AAA or AA size carbon rods?
Used that to titrate the unknown acid, in a 50 ml beaker, with the indicator being no more bubbles and metal left over. 40 ml of spent acid from balloon job had room for 0.5 g more zinc. 40 ml of "fresh" acid dissolved 4.0 g. That works out to 0.19 and 1.53 mol/L of Zn++.
The useful difference, 1.34 mol/L, closely matches the result from balloon job numbers: 38.9 g metal into 0.45 L of acid -> 1.32 mol/L. So it was fair to regard the die-cast scrap as zinc.
It also tells us that the acid had about 1.53 x 2 = 3.06 mol/L of H+ to begin with. That's 1/4 as strong as muriatic acid from the store. So I must have diluted it with 3 parts of water, not 4, when preparing to de-scale some hot rolled steel many months ago. (not the round parts in Pickling thread here.) Seemed to be a good concentration for well behaved H2 generation.
Couple of other interesting details. The liquid had a strong yellow color, which practically disappeared as the zinc reaction approached its end. And the black debris wasn't just tarry residue from battery zinc. It seemed to grow in dendrite form on the last piece of metal, and it was drawn toward a magnet.


That open vessel of hydrochloric acid didn't spend much time on or around the scale. The system lost a total of about 2.6 g, probably mostly as water vapor and maybe some molecular HCl. At cleanup time, the optical refraction contrast between zinc liquor and plain water was strong.
klugesmith, Mon Jun 25 2018, 03:48PM
Bringing back childhood memories, I got some pure zinc metal by taking apart batteries. What was left of it, in cells from the b-waste bin. Anybody need some AAA or AA size carbon rods?
Used that to titrate the unknown acid, in a 50 ml beaker, with the indicator being no more bubbles and metal left over. 40 ml of spent acid from balloon job had room for 0.5 g more zinc. 40 ml of "fresh" acid dissolved 4.0 g. That works out to 0.19 and 1.53 mol/L of Zn++.
The useful difference, 1.34 mol/L, closely matches the result from balloon job numbers: 38.9 g metal into 0.45 L of acid -> 1.32 mol/L. So it was fair to regard the die-cast scrap as zinc.
It also tells us that the acid had about 1.53 x 2 = 3.06 mol/L of H+ to begin with. That's 1/4 as strong as muriatic acid from the store. So I must have diluted it with 3 parts of water, not 4, when preparing to de-scale some hot rolled steel many months ago. (not the round parts in Pickling thread here.) Seemed to be a good concentration for well behaved H2 generation.
Couple of other interesting details. The liquid had a strong yellow color, which practically disappeared as the zinc reaction approached its end. And the black debris wasn't just tarry residue from battery zinc. It seemed to grow in dendrite form on the last piece of metal, and it was drawn toward a magnet.



That open vessel of hydrochloric acid didn't spend much time on or around the scale. The system lost a total of about 2.6 g, probably mostly as water vapor and maybe some molecular HCl. At cleanup time, the optical refraction contrast between zinc liquor and plain water was strong.
Re: Preparation of dry hydrogen?
Plasma, Fri Jun 29 2018, 08:03PM
Kludgesmith if you need a high purity Zn source, you can go to a engineering supplier store and ask for glav stick, they are about 10mm square by 300mm,its used by a gas torch to heat steel and the stick is pressed against the hot steel
Plasma, Fri Jun 29 2018, 08:03PM
Kludgesmith if you need a high purity Zn source, you can go to a engineering supplier store and ask for glav stick, they are about 10mm square by 300mm,its used by a gas torch to heat steel and the stick is pressed against the hot steel
Re: Preparation of dry hydrogen?
johnf, Fri Jun 29 2018, 08:34PM
Plasma
WRONG
I know you are in NZ and galv stick here is pure zinc with pure indium added so it will stick to steel its also why it is very expensive compared to pure zinc.
The best source of pure zinc is boat anodes as the zinc has to be better than 99% to continue working in saltwaqter properly.
Also plumbers make flashings from pure zinc sheet just ask you will get some offcuts for nix
johnf, Fri Jun 29 2018, 08:34PM
Plasma
WRONG
I know you are in NZ and galv stick here is pure zinc with pure indium added so it will stick to steel its also why it is very expensive compared to pure zinc.
The best source of pure zinc is boat anodes as the zinc has to be better than 99% to continue working in saltwaqter properly.
Also plumbers make flashings from pure zinc sheet just ask you will get some offcuts for nix
Re: Preparation of dry hydrogen?
Plasma, Sat Jun 30 2018, 12:38AM
Thanks johnf, I wasn't 100% sure of the purity, do you mean calvnic protection...
edit:car radotors are Mg, from a recycling centre you could pick them up for $10
Plasma, Sat Jun 30 2018, 12:38AM
Thanks johnf, I wasn't 100% sure of the purity, do you mean calvnic protection...
edit:car radotors are Mg, from a recycling centre you could pick them up for $10
Print this page