
Cooling with liquid CO2
Dr. Slack, Tue Aug 15 2017, 11:46AMI'm trying to compare the cooling effects of ice and liquid CO2, to see whether a cold box based on bottled CO2 release would be at all practical/affordable compared with ice. I am having a hard time trying to get believable data about the Joule Thompson expansion of the gaseous CO2 after vapourisation.
I'm assuming the box would run at 0C.
The calculation for ice is easy, the latent heat of fusion is about 333J/g (wikipedia).
I'm assuming the cylinder and a significant length of post-nozzle tube would be entirely contained in the box, and that gas would be released at a slow flow through a small nozzle, so that liquid and gas would stay at about 0C. AFAICS, it is a two stage process. Initially, liquid at 0C 34bar boils to gas at 0C 34bar, absorbing about 230J/g (CRC handbook via wikipedia, latent heat given with respect to 0C 34bar conditions for both liquid and gas). So far, so good.
Then the gas has to go from 34 bar to 1 bar, increasing in volume, for simplicity though a nozzle rather than an engine. This should be covered by the J-T coefficient, though I see contradictory figures for the coefficient depending on pressure ratios. Are there any figures available for the specific 34bar to 1 bar 0C conditions?
Any extra heat absorbed should be in addition to the 230J/g vapourisation, bringing the W/W cooling closer to the figure for ice? How much heat is absorbed in this expansion phase?
[edit] I've now got some figures for 273K of 1.27 at 1bar, 1.38 at 20 bar, and 1.47 at 34bar, from

, so it looks like it's more or less constant, giving approximately 46 degrees change. Does this sound reasonable? [/edit]
Re: Cooling with liquid CO2
klugesmith, Tue Aug 15 2017, 05:34PM
A subject near & dear to me, Dr Slack. Last Saturday I sprayed some liquid CO2 from an upside-down 20 lb cylinder, to pre-chill a vacuum flask for storing some dry ice. And last night I was looking up high pressure hoses and flare fittings for CO2 at cylinder pressure.
Anyway, problems like you're talking about can often be solved graphically with a P-h chart (Log Pressure - Enthalpy) for the working fluid. Generations ago, these were drawn for most materials used as refrigerants and/or stored as pressurized liquids. E.g.
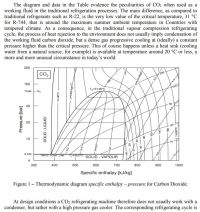
Carbon dioxide is sort of an odd egg, not only because of its low critical temperature but its high triple point pressure. When searching the Internet, after weeding out charts about chemical pH and mineral compositions, the thermodynamic charts for CO2 mostly cut off below 5 bar absolute pressure, when there's no more fluid. Finding one that has numbers for 1 bar pressure is still much easier than the first time I did it (1992, pre-Internet). To save you the trouble, here are one in BTU/lb and one in kJ/kg. The only ones I've ever seen!

klugesmith, Tue Aug 15 2017, 05:34PM
A subject near & dear to me, Dr Slack. Last Saturday I sprayed some liquid CO2 from an upside-down 20 lb cylinder, to pre-chill a vacuum flask for storing some dry ice. And last night I was looking up high pressure hoses and flare fittings for CO2 at cylinder pressure.

Anyway, problems like you're talking about can often be solved graphically with a P-h chart (Log Pressure - Enthalpy) for the working fluid. Generations ago, these were drawn for most materials used as refrigerants and/or stored as pressurized liquids. E.g.


Carbon dioxide is sort of an odd egg, not only because of its low critical temperature but its high triple point pressure. When searching the Internet, after weeding out charts about chemical pH and mineral compositions, the thermodynamic charts for CO2 mostly cut off below 5 bar absolute pressure, when there's no more fluid. Finding one that has numbers for 1 bar pressure is still much easier than the first time I did it (1992, pre-Internet). To save you the trouble, here are one in BTU/lb and one in kJ/kg. The only ones I've ever seen!


Re: Cooling with liquid CO2
johnf, Tue Aug 15 2017, 07:21PM
Dr Slack
I used to have an environmental chamber that went +120C to -70C
The box used a K type thermocouple controller that controlled a heater for hot and a solenoid valve cold. CO2 was spayed onto the inner surface of unit. Worked very well. You need to specify liquid delivery cylinder as once the wrong one was supplied and the unit could not make -10C
johnf, Tue Aug 15 2017, 07:21PM
Dr Slack
I used to have an environmental chamber that went +120C to -70C
The box used a K type thermocouple controller that controlled a heater for hot and a solenoid valve cold. CO2 was spayed onto the inner surface of unit. Worked very well. You need to specify liquid delivery cylinder as once the wrong one was supplied and the unit could not make -10C
Re: Cooling with liquid CO2
klugesmith, Tue Aug 15 2017, 09:37PM
I hope your enthusiasm is enough to go compare those J-T coefficients with the graphical approach.
That would let us check the work of an unnamed chartmaker, using the last of the figures I pointed to.
Here annotated with some points along the Dr Slack path.

Parameters casually eyeballed from chart:
Point 1: saturated liquid at 0 °C, 34 bar. h = -100 J/g
Point 2: saturated vapor at 0 °C, 34 bar. h = +138 J/j
Point 3: superheated vapor at 0 °C, 20 bar. h = +165 J/g
Point 4: superheated vapor at 0 °C, 1 bar. h = +191 J/g.
Total heat absorption up to that point is not far behind the figure you gave for latent heat of fusion in H2O.
One alternate path begins with a step from point 1 to point D. That represents depressurization of liquid from 34 bar at 0 °C to 1 bar, with no exchange of heat, and no mechanical work done by or on the expanding material. Adiabatic and not isentropic. Any mechanical work that might have been done by the expanding fluid was internally converted to heat, through turbulence and friction.
State D is a mixture of solid and vapor at normal dry ice temperature, usually given as -78.5 °C. The mass fraction which is vapor, conventionally called x, looks like about 0.6. The other P-h chart, scaled in psia and BTU, has iso-x lines drawn in the solid/vapor region. Over there it looks like D is about 57% vapor. We could then suck heat along the path from point D to point 4 along the 1-bar pressure line. First evaporate all the solid material, then warm the vapor to 0 °C.
johnf testified:
>>You need to specify liquid delivery cylinder as once the wrong one was supplied and the unit could not make -10C
On the P-h chart, depressurization of vapor from 34 bar and 0°C would take us straight down from point 2. I think with that mistake, most of the chilling goes into liquid CO2 sitting undisturbed in the bottom part cylinder. For Dr Slack's application, with whole cylinder inside the cooled space, I think the end result is the same.
Of course the normal pressure of 1 atmosphere is a little bit more than a bar, unless you are more than 111 meters above sea level. Originally the previous sentence included "or unless you are a follower of IUPAC." Oops, the 1982 change by IUPAC was not to redefine the atm. It was to redefine STP, from P=1 atm to P=1 bar. And don't you forget, the official torr is exactly 101325 / 760 pascals. Anyway...
When pressure is reduced to 1 bar, CO2 (just like water) vapor equilibrium is at a lower temperature. I figure 0.16 °C lower than the literature value. Probably time for a switch to different medication.
klugesmith, Tue Aug 15 2017, 09:37PM
I hope your enthusiasm is enough to go compare those J-T coefficients with the graphical approach.
That would let us check the work of an unnamed chartmaker, using the last of the figures I pointed to.
Here annotated with some points along the Dr Slack path.

Parameters casually eyeballed from chart:
Point 1: saturated liquid at 0 °C, 34 bar. h = -100 J/g
Point 2: saturated vapor at 0 °C, 34 bar. h = +138 J/j
Point 3: superheated vapor at 0 °C, 20 bar. h = +165 J/g
Point 4: superheated vapor at 0 °C, 1 bar. h = +191 J/g.
Total heat absorption up to that point is not far behind the figure you gave for latent heat of fusion in H2O.
One alternate path begins with a step from point 1 to point D. That represents depressurization of liquid from 34 bar at 0 °C to 1 bar, with no exchange of heat, and no mechanical work done by or on the expanding material. Adiabatic and not isentropic. Any mechanical work that might have been done by the expanding fluid was internally converted to heat, through turbulence and friction.
State D is a mixture of solid and vapor at normal dry ice temperature, usually given as -78.5 °C. The mass fraction which is vapor, conventionally called x, looks like about 0.6. The other P-h chart, scaled in psia and BTU, has iso-x lines drawn in the solid/vapor region. Over there it looks like D is about 57% vapor. We could then suck heat along the path from point D to point 4 along the 1-bar pressure line. First evaporate all the solid material, then warm the vapor to 0 °C.
johnf testified:
>>You need to specify liquid delivery cylinder as once the wrong one was supplied and the unit could not make -10C
On the P-h chart, depressurization of vapor from 34 bar and 0°C would take us straight down from point 2. I think with that mistake, most of the chilling goes into liquid CO2 sitting undisturbed in the bottom part cylinder. For Dr Slack's application, with whole cylinder inside the cooled space, I think the end result is the same.
Of course the normal pressure of 1 atmosphere is a little bit more than a bar, unless you are more than 111 meters above sea level. Originally the previous sentence included "or unless you are a follower of IUPAC." Oops, the 1982 change by IUPAC was not to redefine the atm. It was to redefine STP, from P=1 atm to P=1 bar. And don't you forget, the official torr is exactly 101325 / 760 pascals. Anyway...
When pressure is reduced to 1 bar, CO2 (just like water) vapor equilibrium is at a lower temperature. I figure 0.16 °C lower than the literature value. Probably time for a switch to different medication.

Re: Cooling with liquid CO2
Dr. Slack, Wed Aug 16 2017, 10:53AM
... to finish the calculation I started doing with the J-T coefficient, the 46 degrees of cooling corresponds to a further absorbtion of 38J/g (via Cp), giving a total heat absorption of around 270J/g. This compares with the 291J/g from your PE diagram, which is the same ballpark, but still quite a difference. Is my approximation of a constant 1.37K/bar for the J-T coefficient between 34 and 1bar sufficient for the discrepancy? Thanks for your time to dig out, but most importantly to interpret for me, the PE diagram.
What's the name of the line that goes 2-3-4 on your annotated diagram, an iso-what? I will have to go back to my school books and relearn how enthalpy, Gibbs free energy etc. are defined.
Dr. Slack, Wed Aug 16 2017, 10:53AM
... to finish the calculation I started doing with the J-T coefficient, the 46 degrees of cooling corresponds to a further absorbtion of 38J/g (via Cp), giving a total heat absorption of around 270J/g. This compares with the 291J/g from your PE diagram, which is the same ballpark, but still quite a difference. Is my approximation of a constant 1.37K/bar for the J-T coefficient between 34 and 1bar sufficient for the discrepancy? Thanks for your time to dig out, but most importantly to interpret for me, the PE diagram.
What's the name of the line that goes 2-3-4 on your annotated diagram, an iso-what? I will have to go back to my school books and relearn how enthalpy, Gibbs free energy etc. are defined.
Re: Cooling with liquid CO2
Sulaiman, Wed Aug 16 2017, 05:06PM
klugesmith,
me too, thanks for the lesson, and the graph.
Sulaiman, Wed Aug 16 2017, 05:06PM
klugesmith,
me too, thanks for the lesson, and the graph.
Print this page