
Vacuum system for gas discharge experiments
Physikfan, Mon Apr 24 2017, 04:48PMI have built up a vacuum system for gas discharge tests.
It consists of a two-stage rotary vane pump Leybold D2A, with 240 V single-phase, with new oil filling, the oil change was not a pleasant work.
The previous minimum pressure is controlled by the two electronic manometers 4 x 10 ** - 2 mbar after approximately 20 minutes of operation of the pump.

Can someone give me a hint how I could get a better vacuum?
Re: Vacuum system for gas discharge experiments
Proud Mary, Mon Apr 24 2017, 06:58PM
Hello Physikfan! :)
1) If you want to evacuate a glass vessel, you could place a getter inside, and then activate it when you have got to the lowest pressure that you can get with the pump. Fox example, if you put some magnesium or calcium metal on the inside of the glass, when you heat it from outside, the metals will form their oxides, and so remove oxygen from the gas remaining in the system. This is what they do with thermionic valves (US: tubes)
2. If you first flush your system with helium balloon gas, and then evacuate it, you won't get a lower pressure, but you will get a longer electron free path useful in many experiments
Proud Mary, Mon Apr 24 2017, 06:58PM
Hello Physikfan! :)
1) If you want to evacuate a glass vessel, you could place a getter inside, and then activate it when you have got to the lowest pressure that you can get with the pump. Fox example, if you put some magnesium or calcium metal on the inside of the glass, when you heat it from outside, the metals will form their oxides, and so remove oxygen from the gas remaining in the system. This is what they do with thermionic valves (US: tubes)
2. If you first flush your system with helium balloon gas, and then evacuate it, you won't get a lower pressure, but you will get a longer electron free path useful in many experiments
Re: Vacuum system for gas discharge experiments
johnf, Mon Apr 24 2017, 07:24PM
That pump should do better than that
Have you gas ballasted the pump for half an hour
When you do you should use an oil mist trap or vent the pump outlet outside.
the pump is working well enough to back either a turbo or diff pump that would get you to 10^-6 millibar easily
johnf, Mon Apr 24 2017, 07:24PM
That pump should do better than that
Have you gas ballasted the pump for half an hour
When you do you should use an oil mist trap or vent the pump outlet outside.
the pump is working well enough to back either a turbo or diff pump that would get you to 10^-6 millibar easily
Re: Vacuum system for gas discharge experiments
klugesmith, Mon Apr 24 2017, 07:28PM
In a chamber that size, 4e-2 mbar (a.k.a. 30 microns) with a 2-stage rotary pump should not be a big disappointment.
What happens if you connect the vacuum gauge, and nothing else, directly to the pump inlet?
If you are lucky, the indicated pressure will be substantially lower than you got in the full system.
Then the system might benefit from having a fatter, shorter suction hose. Are you familiar with the "vacuum conductance" of pipes and hoses?
klugesmith, Mon Apr 24 2017, 07:28PM
In a chamber that size, 4e-2 mbar (a.k.a. 30 microns) with a 2-stage rotary pump should not be a big disappointment.
What happens if you connect the vacuum gauge, and nothing else, directly to the pump inlet?
If you are lucky, the indicated pressure will be substantially lower than you got in the full system.
Then the system might benefit from having a fatter, shorter suction hose. Are you familiar with the "vacuum conductance" of pipes and hoses?
Re: Vacuum system for gas discharge experiments
Sulaiman, Mon Apr 24 2017, 11:57PM
a two-stage rotary should get you to mean free path lengths of 10 to 100 mm
for longer free paths, ion and turbo pumps are good, but expensive,
I've not tried yet but a Sprengel pump looks to be the easiest hobby approach
e.g.
Sulaiman, Mon Apr 24 2017, 11:57PM
a two-stage rotary should get you to mean free path lengths of 10 to 100 mm
for longer free paths, ion and turbo pumps are good, but expensive,
I've not tried yet but a Sprengel pump looks to be the easiest hobby approach

e.g.

Re: Vacuum system for gas discharge experiments
johnf, Tue Apr 25 2017, 02:01AM
By golly
if we where to go with esoteric pumps then the
Toepler pump would be a contender
saw one on a video of home made vacuum tubes by a "french man" i think
johnf, Tue Apr 25 2017, 02:01AM
By golly
if we where to go with esoteric pumps then the
Toepler pump would be a contender
saw one on a video of home made vacuum tubes by a "french man" i think
Re: Vacuum system for gas discharge experiments
Sulaiman, Tue Apr 25 2017, 03:42AM
I am currently looking for 1+ m of 2.5mm i.d. glass tubing to make a Sprengel pump, as I have the other requirements,
I just need to order a sufficient range of tubing to justify postage cost of one tube.
After watching the Cody's Lab video I decided to make one for myself,
a dual-stage rotary for roughing and a Sprengel for the lowest pressures.
(I have no way to measure these pressures yet)
As a hobbyist this seems the cheapest.easiest route for me to get vacuum suitable for 4HV-type experimenting.
The only practical Toepler pumps that I have seen require rather large quantities of mercury, and/or glass float valves,
but if anyone knows of a better diy mPa range pump, please tell us.
... the sooner the better for me.
Sulaiman, Tue Apr 25 2017, 03:42AM
I am currently looking for 1+ m of 2.5mm i.d. glass tubing to make a Sprengel pump, as I have the other requirements,
I just need to order a sufficient range of tubing to justify postage cost of one tube.
After watching the Cody's Lab video I decided to make one for myself,
a dual-stage rotary for roughing and a Sprengel for the lowest pressures.
(I have no way to measure these pressures yet)
As a hobbyist this seems the cheapest.easiest route for me to get vacuum suitable for 4HV-type experimenting.
The only practical Toepler pumps that I have seen require rather large quantities of mercury, and/or glass float valves,
but if anyone knows of a better diy mPa range pump, please tell us.
... the sooner the better for me.
Re: Vacuum system for gas discharge experiments
Physikfan, Tue Apr 25 2017, 09:16AM
Hi Sulaiman
Please, could you show me the link to
"After watching the Cody's Lab video"
Do you have the possibility to do such experiments outdoors or in a glovebox?
As you may know, very small, almost invisible mercury globules can spoil the air in a closed room for years.
As a teenager I once had a mercury poisoning through a broken fever thermometer in the bedroom.
Physikfan, Tue Apr 25 2017, 09:16AM
Hi Sulaiman
Please, could you show me the link to
"After watching the Cody's Lab video"
Do you have the possibility to do such experiments outdoors or in a glovebox?
As you may know, very small, almost invisible mercury globules can spoil the air in a closed room for years.
As a teenager I once had a mercury poisoning through a broken fever thermometer in the bedroom.
Re: Vacuum system for gas discharge experiments
Physikfan, Tue Apr 25 2017, 09:42AM
Another point:
The limiting factor of such pumps is the vapor pressure of the liquid media.
Normal vacuum pump oil about 10 ** - 3 mbar
Mercury at 20 ° C also about 10 ** -3 mbar
Physikfan, Tue Apr 25 2017, 09:42AM
Another point:
The limiting factor of such pumps is the vapor pressure of the liquid media.
Normal vacuum pump oil about 10 ** - 3 mbar
Mercury at 20 ° C also about 10 ** -3 mbar
Re: Vacuum system for gas discharge experiments
Physikfan, Tue Apr 25 2017, 09:56AM
Hi Johnf
Please could you give me the link to
"Toepler pump would be a contender
saw one on a video of home made vacuum tubes by a "french man""
Regards
Physikfan
Physikfan, Tue Apr 25 2017, 09:56AM
Hi Johnf
Please could you give me the link to
"Toepler pump would be a contender
saw one on a video of home made vacuum tubes by a "french man""
Regards
Physikfan
Re: Vacuum system for gas discharge experiments
Sulaiman, Tue Apr 25 2017, 10:33AM
The second link in my first post on this topic, above.
Most experiments like this I do in my alfresco laboratory, recently relocated and repainted.

I doubt that you had significant mercury poisoning due to the vapour from the liquid mercury of one thermometer,
unless you sleep on the floor,
the vapour pressure is so low that mercury evaporates very slowly,
even a little ventilation (opening the door twice a day) should make mercury vapour concentrations negligible.
Were you clinically diagnosed using blood tests ?
(very often chemical exposure can cause psychosomatic symptoms)
If you do have a small mercury spill, use powdered sulphur.
......................................... .................................
"The limiting factor of such pumps is the vapor pressure of the liquid media.
Normal vacuum pump oil about 10 ** - 3 mbar
Mercury at 20 ° C also about 10 ** -3 mbar"
................................................. .........................
True, but rotary pumps do not get down to the vapour pressure of the oil due to mechanical limitations,
and oil instead of mercury for a Sprengel pump would require a very long drop (c11m) due to the difference in density.
Sulaiman, Tue Apr 25 2017, 10:33AM
Physikfan wrote ...
Hi Sulaiman
Please, could you show me the link to
"After watching the Cody's Lab video"
Do you have the possibility to do such experiments outdoors or in a glovebox?
As you may know, very small, almost invisible mercury globules can spoil the air in a closed room for years.
As a teenager I once had a mercury poisoning through a broken fever thermometer in the bedroom.
Hi Sulaiman
Please, could you show me the link to
"After watching the Cody's Lab video"
Do you have the possibility to do such experiments outdoors or in a glovebox?
As you may know, very small, almost invisible mercury globules can spoil the air in a closed room for years.
As a teenager I once had a mercury poisoning through a broken fever thermometer in the bedroom.
The second link in my first post on this topic, above.
Most experiments like this I do in my alfresco laboratory, recently relocated and repainted.

I doubt that you had significant mercury poisoning due to the vapour from the liquid mercury of one thermometer,
unless you sleep on the floor,
the vapour pressure is so low that mercury evaporates very slowly,
even a little ventilation (opening the door twice a day) should make mercury vapour concentrations negligible.
Were you clinically diagnosed using blood tests ?
(very often chemical exposure can cause psychosomatic symptoms)
If you do have a small mercury spill, use powdered sulphur.
......................................... .................................
"The limiting factor of such pumps is the vapor pressure of the liquid media.
Normal vacuum pump oil about 10 ** - 3 mbar
Mercury at 20 ° C also about 10 ** -3 mbar"
................................................. .........................
True, but rotary pumps do not get down to the vapour pressure of the oil due to mechanical limitations,
and oil instead of mercury for a Sprengel pump would require a very long drop (c11m) due to the difference in density.
Re: Vacuum system for gas discharge experiments
Proud Mary, Tue Apr 25 2017, 03:08PM
Mr Physik,
have you heard of The Bell Jar : Vacuum Techniques and Related Topics for the Amateur Investigator?
It is full of good stuff, some of it dating back more than 50 years.

Proud Mary, Tue Apr 25 2017, 03:08PM
Mr Physik,
have you heard of The Bell Jar : Vacuum Techniques and Related Topics for the Amateur Investigator?
It is full of good stuff, some of it dating back more than 50 years.

Re: Vacuum system for gas discharge experiments
johnf, Tue Apr 25 2017, 07:31PM
Heres the link to home made tubes
i'm sure the toepler pump is in there somewhere

johnf, Tue Apr 25 2017, 07:31PM
Heres the link to home made tubes
i'm sure the toepler pump is in there somewhere

Re: Vacuum system for gas discharge experiments
jpsmith123, Tue Apr 25 2017, 07:41PM
Speaking of the Bell Jar, this article may be of interest:

On my mechanical vacuum pump, I use two traps in series; a shredded copper trap to block back-streaming pump oil, followed by a molecular sieve trap, which will trap both oil vapor and water vapor. I also have some Edwards "Ultragrade 19" mechanical pump oil which is supposed to have a relatively low vapor pressure.
I'm not sure how low this system will go, but it rapidly buries my Varian 531 TC gauge; so I think it's well below 0.001 torr.
jpsmith123, Tue Apr 25 2017, 07:41PM
Speaking of the Bell Jar, this article may be of interest:

On my mechanical vacuum pump, I use two traps in series; a shredded copper trap to block back-streaming pump oil, followed by a molecular sieve trap, which will trap both oil vapor and water vapor. I also have some Edwards "Ultragrade 19" mechanical pump oil which is supposed to have a relatively low vapor pressure.
I'm not sure how low this system will go, but it rapidly buries my Varian 531 TC gauge; so I think it's well below 0.001 torr.
Re: Vacuum system for gas discharge experiments
Physikfan, Tue Apr 25 2017, 08:15PM
Hi Sulaiman
I have read about the Sprengel pump in Wikipedia, the free encyclopedia.
"The device was later found capable of reducing the pressure to less than 1 mPa (9.87 x10−9 atm).[5]"
This source [5] is "The New Student's Reference Work/Air Pump"
with the following statement without a further reference:
"A pump of this type is capable of producing a vacuum in which the pressure is only 100,000,000th of an atmosphere."
I cannot believe that a mercury filled Sprengel pump could produce a pressure which is lower by a factor of 100 than the vapor pressure of mercury itself.
Please I am looking forward to all your comments.
Physikfan, Tue Apr 25 2017, 08:15PM
Hi Sulaiman
I have read about the Sprengel pump in Wikipedia, the free encyclopedia.
"The device was later found capable of reducing the pressure to less than 1 mPa (9.87 x10−9 atm).[5]"
This source [5] is "The New Student's Reference Work/Air Pump"
with the following statement without a further reference:
"A pump of this type is capable of producing a vacuum in which the pressure is only 100,000,000th of an atmosphere."
I cannot believe that a mercury filled Sprengel pump could produce a pressure which is lower by a factor of 100 than the vapor pressure of mercury itself.
Please I am looking forward to all your comments.
Re: Vacuum system for gas discharge experiments
Physikfan, Tue Apr 25 2017, 08:29PM
Ad my mercury poisoning as a teenager:
I slept very close to mercury droplets inside a carpet during weeks.
As a consequence I had several times a very large increased salivation which is a typical phenomenon for a mercury poisoning.
Physikfan, Tue Apr 25 2017, 08:29PM
Ad my mercury poisoning as a teenager:
I slept very close to mercury droplets inside a carpet during weeks.
As a consequence I had several times a very large increased salivation which is a typical phenomenon for a mercury poisoning.
Re: Vacuum system for gas discharge experiments
Sulaiman, Tue Apr 25 2017, 11:49PM
Although the vapour pressure of mercury at room temperature is about 1 Pa
it is about 0.1 Pa at 0C and the triple point of mercury is -39 C, 0.2 mPa
and 1/100,000,000 atmosphere = 1 mPa
... seems achievable, but not easy.
Sulaiman, Tue Apr 25 2017, 11:49PM
Physikfan wrote ...
"A pump of this type is capable of producing a vacuum in which the pressure is only 100,000,000th of an atmosphere."
I cannot believe that a mercury filled Sprengel pump could produce a pressure which is lower by a factor of 100 than the vapor pressure of mercury itself.
Please I am looking forward to all your comments.
"A pump of this type is capable of producing a vacuum in which the pressure is only 100,000,000th of an atmosphere."
I cannot believe that a mercury filled Sprengel pump could produce a pressure which is lower by a factor of 100 than the vapor pressure of mercury itself.
Please I am looking forward to all your comments.
Although the vapour pressure of mercury at room temperature is about 1 Pa
it is about 0.1 Pa at 0C and the triple point of mercury is -39 C, 0.2 mPa
and 1/100,000,000 atmosphere = 1 mPa
... seems achievable, but not easy.
Re: Vacuum system for gas discharge experiments
Physikfan, Wed Apr 26 2017, 12:53PM
I have used a vapor pressure calculator

to compute a few vapor pressure values for Hg and Ga:
Hg:
T(K)___T(°C)_p(mbar)
233.2 - 40.00
238.2 -35.00 5.33e-6
243.2 -30.00 1.01e- 5
248.2 -25.00 1.85e-5
253.2 -20.00 3.31e- 5
258.1 -15.00 5.81e-5
263.1 -10.00 9.98e- 5
268.1 -5.000 1.68e-4
273.1 0.000 2.77e- 4
278.1 5.000 4.50e-4
283.1 10.00 7.17e- 4
288.1 15.00 1.12e-3
293.1 20.00 1.74e- 3
298.1 25.00 2.64e-3
303.1 30.00 3.97e- 3
308.1 35.00 5.88e-3
313.1 40.00 8.61e- 3
318.1 45.00 0.0124
323.1 50.00 0.0178
Ga:
T(K) __T(°C)__p(mbar)
303.1 30.00 6.08e- 38
308.1 35.00 3.39e-37
313.1 40.00 1.79e- 36
318.1 45.00 8.94e-36
323.1 50.00 4.26e-35
At the moment the vapor pressure values for gallium seem to me extremely low, even uncredibly low.
I will try to find real scientific papers on vapor pressures of liquid gallium to verify these numbers above.
For 100 g gallium I found prices around 50 Euro at ebay.
The question is:
How much liquid gallium is necessary to realise a Sprengel pump with liquid gallium at 40°C?
Physikfan, Wed Apr 26 2017, 12:53PM
I have used a vapor pressure calculator

to compute a few vapor pressure values for Hg and Ga:
Hg:
T(K)___T(°C)_p(mbar)
233.2 - 40.00
238.2 -35.00 5.33e-6
243.2 -30.00 1.01e- 5
248.2 -25.00 1.85e-5
253.2 -20.00 3.31e- 5
258.1 -15.00 5.81e-5
263.1 -10.00 9.98e- 5
268.1 -5.000 1.68e-4
273.1 0.000 2.77e- 4
278.1 5.000 4.50e-4
283.1 10.00 7.17e- 4
288.1 15.00 1.12e-3
293.1 20.00 1.74e- 3
298.1 25.00 2.64e-3
303.1 30.00 3.97e- 3
308.1 35.00 5.88e-3
313.1 40.00 8.61e- 3
318.1 45.00 0.0124
323.1 50.00 0.0178
Ga:
T(K) __T(°C)__p(mbar)
303.1 30.00 6.08e- 38
308.1 35.00 3.39e-37
313.1 40.00 1.79e- 36
318.1 45.00 8.94e-36
323.1 50.00 4.26e-35
At the moment the vapor pressure values for gallium seem to me extremely low, even uncredibly low.
I will try to find real scientific papers on vapor pressures of liquid gallium to verify these numbers above.
For 100 g gallium I found prices around 50 Euro at ebay.
The question is:
How much liquid gallium is necessary to realise a Sprengel pump with liquid gallium at 40°C?
Re: Vacuum system for gas discharge experiments
Physikfan, Wed Apr 26 2017, 01:33PM
Here are another sources for the vapor pressure of gallium:


"This very efficient cooling and the very low vapor pressure for
liquid gallium (less than 10**-12 Torr at 100°C) make liquid gallium a very
attractive cooling fluid for high vacuum synchrotron applications."
Physikfan, Wed Apr 26 2017, 01:33PM
Here are another sources for the vapor pressure of gallium:


"This very efficient cooling and the very low vapor pressure for
liquid gallium (less than 10**-12 Torr at 100°C) make liquid gallium a very
attractive cooling fluid for high vacuum synchrotron applications."
Re: Vacuum system for gas discharge experiments
Sulaiman, Wed Apr 26 2017, 04:44PM
(smaller = slower pumping, larger - air able to 'bubble' up past the mercury - not trapped)
so using id = 2.5 mm and drop tube = 1000 mm, volume = 4.9 ml maximum in the tube,
so probably at least 100 g... if mercury.
as gallium is 5.91/13.54 the density of mercury,
the drop tube would need to be > 0.76 x 13.54/5.91 = 1.74 metres MINIMUM,
probably at least 2m required.
If the id is the same then the mass is the same as for mercury, or any other fluid.
(pressure, area and gravity fixed, therefore mass also fixed)
I don't know if it is significant; gallium wets glass and most other materials, mercury does not.
Sulaiman, Wed Apr 26 2017, 04:44PM
Physikfan wrote ...
The question is:
How much liquid gallium is necessary to realise a Sprengel pump with liquid gallium at 40°C?
In Sprengel's original document he mentions that for mercury, 2.5 to 2.75 mm i.d. is optimum,The question is:
How much liquid gallium is necessary to realise a Sprengel pump with liquid gallium at 40°C?
(smaller = slower pumping, larger - air able to 'bubble' up past the mercury - not trapped)
so using id = 2.5 mm and drop tube = 1000 mm, volume = 4.9 ml maximum in the tube,
so probably at least 100 g... if mercury.
as gallium is 5.91/13.54 the density of mercury,
the drop tube would need to be > 0.76 x 13.54/5.91 = 1.74 metres MINIMUM,
probably at least 2m required.
If the id is the same then the mass is the same as for mercury, or any other fluid.
(pressure, area and gravity fixed, therefore mass also fixed)
I don't know if it is significant; gallium wets glass and most other materials, mercury does not.
Re: Vacuum system for gas discharge experiments
Physikfan, Wed Apr 26 2017, 05:11PM
For using gallium
1. the very low vapor pressure
2. it is not that toxic compared to mercury
Against using gallium
1. the high price
2. working temperature above 30°C
You should work with the Sprengel pump in Spain or Greece during summer time!
Physikfan, Wed Apr 26 2017, 05:11PM
For using gallium
1. the very low vapor pressure
2. it is not that toxic compared to mercury
Against using gallium
1. the high price
2. working temperature above 30°C
You should work with the Sprengel pump in Spain or Greece during summer time!
Re: Vacuum system for gas discharge experiments
Physikfan, Wed Apr 26 2017, 05:43PM
Hi jpsmith123 and klugesmith
"I also have some Edwards "Ultragrade 19" mechanical pump oil which is supposed to have a relatively low vapor pressure.
I'm not sure how low this system will go, but it rapidly buries my Varian 531 TC gauge; so I think it's well below 0.001 torr."
You are using a very good vacuum oil, Edwards "Ultragrade 19", about 25 US $/liter:
vapor pressure (mbar):
20 °C 1 x 10**-8
100 °C 1,0 x 10**-3
"What happens if you connect the vacuum gauge, and nothing else, directly to the pump inlet?"
I have done this experiment.
I got 3 x 10**-2 mbar with gas ballast and
I got 2 x 10**-2 mbar without gas ballast.
Physikfan, Wed Apr 26 2017, 05:43PM
Hi jpsmith123 and klugesmith
"I also have some Edwards "Ultragrade 19" mechanical pump oil which is supposed to have a relatively low vapor pressure.
I'm not sure how low this system will go, but it rapidly buries my Varian 531 TC gauge; so I think it's well below 0.001 torr."
You are using a very good vacuum oil, Edwards "Ultragrade 19", about 25 US $/liter:
vapor pressure (mbar):
20 °C 1 x 10**-8
100 °C 1,0 x 10**-3
"What happens if you connect the vacuum gauge, and nothing else, directly to the pump inlet?"
I have done this experiment.
I got 3 x 10**-2 mbar with gas ballast and
I got 2 x 10**-2 mbar without gas ballast.
Re: Vacuum system for gas discharge experiments
klugesmith, Sat Apr 29 2017, 07:41PM
That suggests that your chamber vacuum might be improved by a factor of about two, at best, by using a fatter pipe and reducing leaks. JohnF says something isn't working as well as it should -- that could be the pump or the gauge. Don't you have other pumps?
Sulaiman:
Nice lab station you got there. Thanks for sharing.
It's great idea: to enhance a Sprengel pump by keeping the mercury cold. Never thought of that! Aside from substantially lower vapor pressure, the increased liquid density doesn't hurt.
Someone mentioned that working fluids less dense than mercury would require taller columns. Yes indeed, but nobody said a Sprengel pump needs to discharge directly to atmospheric pressure. A mechanical backing pump, even (say) a diaphragm pump, would substantially reduce the physical height requirement for any fluid. Always consider where the fluid will go in a hardware failure event.
Physikfan, your vapor pressure tables beat me to the punch! I was studying that, and making charts, and got in trouble for coming home late. Had just learned that most materials have a nearly linear relationship between the logarithm of vapor pressure and the inverse of absolute temperature. Here are some data points for Hg from several online references, including the tabular data you posted:
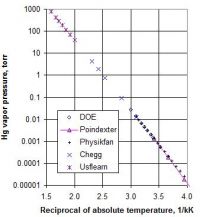
It was also news to me that gallium has a very low vapor pressure, esp. since it seems to have the second lowest MP of all metallic elements. One ref says its BP/MP ratio (in absolute temperature) is the only one higher than 8. That would have to be excepting helium . Anyway, something about atomic bonding makes many high-MP elements relatively volatile, and low-MP elements very nonvolatile in the vapor pressure sense.
Anyway, something about atomic bonding makes many high-MP elements relatively volatile, and low-MP elements very nonvolatile in the vapor pressure sense.
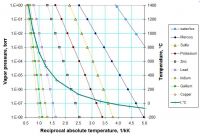
I hope my pretty chart is self-explanatory. Look how indium is way down there in vapor pressure. And how zinc vapor pressure is many orders of magnitude higher than that of lead, in spite of Zn being much harder to melt in a pot on a stove.
As for gallium in Sprengel pumps kept warm, there are also its lower-MP alloys (e.g. galinstan (tm) ) that are used in fever thermometers.
klugesmith, Sat Apr 29 2017, 07:41PM
Physikfan wrote ...
..."What happens if you connect the vacuum gauge, and nothing else, directly to the pump inlet?"
I have done this experiment.
I got 3 x 10**-2 mbar with gas ballast and
I got 2 x 10**-2 mbar without gas ballast.
and, If I recall correctly, 4 or 5e-2 mbar with gauge on the chamber. ..."What happens if you connect the vacuum gauge, and nothing else, directly to the pump inlet?"
I have done this experiment.
I got 3 x 10**-2 mbar with gas ballast and
I got 2 x 10**-2 mbar without gas ballast.
That suggests that your chamber vacuum might be improved by a factor of about two, at best, by using a fatter pipe and reducing leaks. JohnF says something isn't working as well as it should -- that could be the pump or the gauge. Don't you have other pumps?
Sulaiman:
Nice lab station you got there. Thanks for sharing.
It's great idea: to enhance a Sprengel pump by keeping the mercury cold. Never thought of that! Aside from substantially lower vapor pressure, the increased liquid density doesn't hurt.

Someone mentioned that working fluids less dense than mercury would require taller columns. Yes indeed, but nobody said a Sprengel pump needs to discharge directly to atmospheric pressure. A mechanical backing pump, even (say) a diaphragm pump, would substantially reduce the physical height requirement for any fluid. Always consider where the fluid will go in a hardware failure event.
Physikfan, your vapor pressure tables beat me to the punch! I was studying that, and making charts, and got in trouble for coming home late. Had just learned that most materials have a nearly linear relationship between the logarithm of vapor pressure and the inverse of absolute temperature. Here are some data points for Hg from several online references, including the tabular data you posted:

It was also news to me that gallium has a very low vapor pressure, esp. since it seems to have the second lowest MP of all metallic elements. One ref says its BP/MP ratio (in absolute temperature) is the only one higher than 8. That would have to be excepting helium .
 Anyway, something about atomic bonding makes many high-MP elements relatively volatile, and low-MP elements very nonvolatile in the vapor pressure sense.
Anyway, something about atomic bonding makes many high-MP elements relatively volatile, and low-MP elements very nonvolatile in the vapor pressure sense.
I hope my pretty chart is self-explanatory. Look how indium is way down there in vapor pressure. And how zinc vapor pressure is many orders of magnitude higher than that of lead, in spite of Zn being much harder to melt in a pot on a stove.
As for gallium in Sprengel pumps kept warm, there are also its lower-MP alloys (e.g. galinstan (tm) ) that are used in fever thermometers.
Re: Vacuum system for gas discharge experiments
Physikfan, Sun Apr 30 2017, 07:42PM
klugesmith wrote:
"I got 3 x 10**-2 mbar with gas ballast and
I got 2 x 10**-2 mbar without gas ballast.
and, If I recall correctly, 4 or 5e-2 mbar with gauge on the chamber.
That suggests that your chamber vacuum might be improved by a factor of about two, at best, by using a fatter pipe and reducing leaks. JohnF says something isn't working as well as it should -- that could be the pump or the gauge. Don't you have other pumps?"
At the moment I have no other pump.
The pressure gauges seem to be okay. Both meters show the same values as can be seen at my picture in my first posting.
Please, which software have you used for creating these interesting figures on vapor pressures?
Ad Proud Mary
"Mr Physik,
have you heard of The Bell Jar : Vacuum Techniques and Related Topics for the Amateur Investigator?
It is full of good stuff, some of it dating back more than 50 years. "
I think this i a very good stuff, it costs about 80 US$.
Physikfan, Sun Apr 30 2017, 07:42PM
klugesmith wrote:
"I got 3 x 10**-2 mbar with gas ballast and
I got 2 x 10**-2 mbar without gas ballast.
and, If I recall correctly, 4 or 5e-2 mbar with gauge on the chamber.
That suggests that your chamber vacuum might be improved by a factor of about two, at best, by using a fatter pipe and reducing leaks. JohnF says something isn't working as well as it should -- that could be the pump or the gauge. Don't you have other pumps?"
At the moment I have no other pump.
The pressure gauges seem to be okay. Both meters show the same values as can be seen at my picture in my first posting.
Please, which software have you used for creating these interesting figures on vapor pressures?
Ad Proud Mary
"Mr Physik,
have you heard of The Bell Jar : Vacuum Techniques and Related Topics for the Amateur Investigator?
It is full of good stuff, some of it dating back more than 50 years. "
I think this i a very good stuff, it costs about 80 US$.
Re: Vacuum system for gas discharge experiments
klugesmith, Mon May 01 2017, 07:44PM
There are free / open source software alternatives. e.g. gnuplot (from long ago). There are non-free programs, e.g. Matlab, that are much more versatile than Excel for rendering scientific data.
Excel has no vapor pressure feature, AFAIK. All chart values were typed in by hand, or cut and pasted from internet pages, or generated from other spreadsheet cells using simple formulas. For example, the multi-element chart worksheet begins with:
All chart values were typed in by hand, or cut and pasted from internet pages, or generated from other spreadsheet cells using simple formulas. For example, the multi-element chart worksheet begins with:

One data point was obviously wrong in the chart. Manually adding a minus sign, missing in the source, brought the errant datum into line.
Paul F. Dietz, writing in 2000, includes both mercury and lead among elements that have very low vapor pressures at their melting points.
includes both mercury and lead among elements that have very low vapor pressures at their melting points.

By that measure, copper (bottom-most in my chart) is above mercury by a factor of 150.
klugesmith, Mon May 01 2017, 07:44PM
Physikfan wrote ...
Please, which software have you used for creating these interesting figures on vapor pressures?
The charts were made in Microsoft's Excel program. A tool I use every day at work; both the 2003 version and a "2007 or later" version. The latter can handle bigger arrays. But they changed the GUI extensively, in ways that make it horribly cumbersome to produce charts styled the way I want. The defaults seem to reflect the MS Office concept of marketing presentation trends around 2007. Please, which software have you used for creating these interesting figures on vapor pressures?
There are free / open source software alternatives. e.g. gnuplot (from long ago). There are non-free programs, e.g. Matlab, that are much more versatile than Excel for rendering scientific data.
Excel has no vapor pressure feature, AFAIK.
 All chart values were typed in by hand, or cut and pasted from internet pages, or generated from other spreadsheet cells using simple formulas. For example, the multi-element chart worksheet begins with:
All chart values were typed in by hand, or cut and pasted from internet pages, or generated from other spreadsheet cells using simple formulas. For example, the multi-element chart worksheet begins with:
One data point was obviously wrong in the chart. Manually adding a minus sign, missing in the source, brought the errant datum into line.
Paul F. Dietz, writing in 2000,
 includes both mercury and lead among elements that have very low vapor pressures at their melting points.
includes both mercury and lead among elements that have very low vapor pressures at their melting points.
By that measure, copper (bottom-most in my chart) is above mercury by a factor of 150.
Print this page