
Energy Density and Conversion.
Patrick, Sun Jan 22 2017, 01:57AMI need help these units of energy, power and time have my brain all upside down.
If I have 300ml of methanol which is 0.0364 M joules per Liter, (and assuming 100% efficiency) for how many seconds can I get 300 watts ?
starting here, 1 L / 0.3 L = 3.3 then,
0.0364 MJ / 3.3 = 0.011 MJ available in the 300ml.
next 0.011 MJ = 11,000 J.
And 11,000 J / 300 W = 36 seconds is totally wrong.
This dimensional analysis is driving me nuts. Its right in front of me too, i just know it. theres a time value missing i think.
Re: Energy Density and Conversion.
Plasma, Sun Jan 22 2017, 03:23AM
Patrick
Are you sure 0.036MJ/kg is correct, most fuels seem to be around 1-20MJ/kg.
I tried looking on engineering toolbox website but they didn't have methanol.
Plasma, Sun Jan 22 2017, 03:23AM
Patrick
Are you sure 0.036MJ/kg is correct, most fuels seem to be around 1-20MJ/kg.
I tried looking on engineering toolbox website but they didn't have methanol.
Re: Energy Density and Conversion.
Bjørn, Sun Jan 22 2017, 03:24AM
I got the same result, your value for energy content of your methanol looks to be completely wrong, unless it is diluted to the extreme.
Bjørn, Sun Jan 22 2017, 03:24AM
I got the same result, your value for energy content of your methanol looks to be completely wrong, unless it is diluted to the extreme.
Re: Energy Density and Conversion.
Patrick, Sun Jan 22 2017, 03:31AM
 the great all knowing wiki doesnt lie. bow to its greatness. submit to its power.
the great all knowing wiki doesnt lie. bow to its greatness. submit to its power.
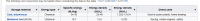
mmmm apparently if i use the wrong numbers i get the wrong answer. Methane and Methanol perhaps are different chemicals.
Patrick, Sun Jan 22 2017, 03:31AM
 the great all knowing wiki doesnt lie. bow to its greatness. submit to its power.
the great all knowing wiki doesnt lie. bow to its greatness. submit to its power.mmmm apparently if i use the wrong numbers i get the wrong answer. Methane and Methanol perhaps are different chemicals.

Re: Energy Density and Conversion.
Bjørn, Sun Jan 22 2017, 03:39AM
You used the value for methane instead of for methanol.
Bjørn, Sun Jan 22 2017, 03:39AM
You used the value for methane instead of for methanol.
Re: Energy Density and Conversion.
Patrick, Sun Jan 22 2017, 03:41AM
i just realized that !
Patrick, Sun Jan 22 2017, 03:41AM
i just realized that !
Re: Energy Density and Conversion.
Patrick, Mon Jan 23 2017, 04:14AM
Ok lets try this with a clear mind.
15.6 MJ / 3.3 = 5.2 MJ available in 300mL
5.2 MJ / 300 W = 17333 / 3600 sec/Hr = 4.8 hours.
4.8 Hr x 0.25 (%) = 1.2 hours.
this seems right ? I think.
Patrick, Mon Jan 23 2017, 04:14AM
Ok lets try this with a clear mind.
15.6 MJ / 3.3 = 5.2 MJ available in 300mL
5.2 MJ / 300 W = 17333 / 3600 sec/Hr = 4.8 hours.
4.8 Hr x 0.25 (%) = 1.2 hours.
this seems right ? I think.
Print this page