
Downs Cell Electrical Insulator
Thomas W, Thu Feb 05 2015, 10:38PMI'm currently looking into building a small Downs Cell for making Sodium metal, mostly for the sake of seeing if I can do it.
the problem I have is trying to find a suitable insulator for the terminals that won't be effected by the high temperature of molten NaCl
Does anyone have a good idea as to what I could use?
thanks.
Thomas
Re: Downs Cell Electrical Insulator
Sulaiman, Thu Feb 05 2015, 11:35PM
seems complicated
for sodium metal you could try something like this
this video shows sodium by electrolysis with an easily available electrode/crucible
Sulaiman, Thu Feb 05 2015, 11:35PM
seems complicated
for sodium metal you could try something like this

this video shows sodium by electrolysis with an easily available electrode/crucible

Re: Downs Cell Electrical Insulator
Thomas W, Thu Feb 05 2015, 11:55PM
That's true, however It's not very pure... and not quite as fun!
The concept of a high temperature insulator is the main issue...
Ideally it needs to not get corroded by molten salt and be able to withstand temperatures of over 800 Degrees *C
Other then that, no problem!
Thomas W, Thu Feb 05 2015, 11:55PM
That's true, however It's not very pure... and not quite as fun!
The concept of a high temperature insulator is the main issue...
Ideally it needs to not get corroded by molten salt and be able to withstand temperatures of over 800 Degrees *C
Other then that, no problem!
Re: Downs Cell Electrical Insulator
Sulaiman, Fri Feb 06 2015, 01:17AM
added one url above
Sodium metal on eBay
hardly seems worth the effort, except for the learning experience of course.
Sulaiman, Fri Feb 06 2015, 01:17AM
added one url above
Sodium metal on eBay

hardly seems worth the effort, except for the learning experience of course.
Re: Downs Cell Electrical Insulator
Thomas W, Fri Feb 06 2015, 08:29AM
To the same degree it hardly seems worth building a go cart, tesla coil, your own house etc.. as someone else has done it for you.

Thomas W, Fri Feb 06 2015, 08:29AM
Sulaiman wrote ...
added one url above
Sodium metal on eBay
hardly seems worth the effort, except for the learning experience of course.
added one url above
Sodium metal on eBay

hardly seems worth the effort, except for the learning experience of course.
To the same degree it hardly seems worth building a go cart, tesla coil, your own house etc.. as someone else has done it for you.

Re: Downs Cell Electrical Insulator
Sulaiman, Fri Feb 06 2015, 11:09AM
for small quantities could you just use a borosilicate/pyrex 'U' tube
and insert anode and cathode from above, one each side?
you could use nichrome wire for electrical heating and possibly for both electrodes,
Wikipedia: Na melts at 98C, NaOH at 318C, Nichrome at 1400C and borosilicate softens at 830C, .... should be do-able
when the process is finished just smash the cheap/disposable tube under oil to retrieve the sodium for refining.
I have not tried, just an idea.
making or using large quantities of sodium is of dubious benefit
Did you look at the second youtube video that I pointed to?
it shows a simple ceramic crucible with electrodes.
Sulaiman, Fri Feb 06 2015, 11:09AM
for small quantities could you just use a borosilicate/pyrex 'U' tube
and insert anode and cathode from above, one each side?
you could use nichrome wire for electrical heating and possibly for both electrodes,
Wikipedia: Na melts at 98C, NaOH at 318C, Nichrome at 1400C and borosilicate softens at 830C, .... should be do-able
when the process is finished just smash the cheap/disposable tube under oil to retrieve the sodium for refining.
I have not tried, just an idea.
making or using large quantities of sodium is of dubious benefit

Did you look at the second youtube video that I pointed to?
it shows a simple ceramic crucible with electrodes.
Re: Downs Cell Electrical Insulator
Wastrel, Fri Feb 06 2015, 02:54PM
Here be dragons. I recommend reading the sodium thread at sciencemadness from start to end.
Aside from chlorine being a war gas at the melting point of sodium chloride sodium metal has a vapour pressure of about a third of an atmosphere.
Molten sodium hydroxide eats glass.
Wastrel, Fri Feb 06 2015, 02:54PM
Here be dragons. I recommend reading the sodium thread at sciencemadness from start to end.
Aside from chlorine being a war gas at the melting point of sodium chloride sodium metal has a vapour pressure of about a third of an atmosphere.
Molten sodium hydroxide eats glass.
Re: Downs Cell Electrical Insulator
Sulaiman, Fri Feb 06 2015, 03:05PM
Molten sodium hydroxide eats glass.
........................................... .
also, molten sodium eats glass
D'oh ! ....... sorry ! a completely stupid idea
Sulaiman, Fri Feb 06 2015, 03:05PM
Molten sodium hydroxide eats glass.
........................................... .
also, molten sodium eats glass
D'oh ! ....... sorry ! a completely stupid idea

Re: Downs Cell Electrical Insulator
Thomas W, Fri Feb 06 2015, 07:45PM
Ah yes, that is not the same method as I want to go for, It is much less pure. I specifically want to use Molten Sodium Chloride so I can get the pure Chlorine and Sodium from the electrolysis I intend to use the Chlorine to make Hydrochloric acid & Hydrogen Peroxide(why not?) and the Sodium for fun / other science stuff.
I intend to have a stainless steel setup made by a machine shop next door who can do it for me cheaply.

Thomas W, Fri Feb 06 2015, 07:45PM
Sulaiman wrote ...
for small quantities could you just use a borosilicate/pyrex 'U' tube
and insert anode and cathode from above, one each side?
you could use nichrome wire for electrical heating and possibly for both electrodes,
Wikipedia: Na melts at 98C, NaOH at 318C, Nichrome at 1400C and borosilicate softens at 830C, .... should be do-able
when the process is finished just smash the cheap/disposable tube under oil to retrieve the sodium for refining.
I have not tried, just an idea.
making or using large quantities of sodium is of dubious benefit
Did you look at the second youtube video that I pointed to?
it shows a simple ceramic crucible with electrodes.
for small quantities could you just use a borosilicate/pyrex 'U' tube
and insert anode and cathode from above, one each side?
you could use nichrome wire for electrical heating and possibly for both electrodes,
Wikipedia: Na melts at 98C, NaOH at 318C, Nichrome at 1400C and borosilicate softens at 830C, .... should be do-able
when the process is finished just smash the cheap/disposable tube under oil to retrieve the sodium for refining.
I have not tried, just an idea.
making or using large quantities of sodium is of dubious benefit

Did you look at the second youtube video that I pointed to?
it shows a simple ceramic crucible with electrodes.
Ah yes, that is not the same method as I want to go for, It is much less pure. I specifically want to use Molten Sodium Chloride so I can get the pure Chlorine and Sodium from the electrolysis I intend to use the Chlorine to make Hydrochloric acid & Hydrogen Peroxide(why not?) and the Sodium for fun / other science stuff.
I intend to have a stainless steel setup made by a machine shop next door who can do it for me cheaply.

Re: Downs Cell Electrical Insulator
Ash Small, Fri Feb 06 2015, 11:13PM
Chlorine is THE worst enemy of stainless steel, especially 304.
Liquid sodium 'may' dissolve some of the metals in the alloy, not sure. (molten aluminium dissolves a bit of iron, for example.)
Ash Small, Fri Feb 06 2015, 11:13PM
Chlorine is THE worst enemy of stainless steel, especially 304.
Liquid sodium 'may' dissolve some of the metals in the alloy, not sure. (molten aluminium dissolves a bit of iron, for example.)
Re: Downs Cell Electrical Insulator
Thomas W, Fri Feb 06 2015, 11:14PM
Hmm, I guess it's Iron or mild steel then, not sure what they actually make it out of.
Thomas W, Fri Feb 06 2015, 11:14PM
Hmm, I guess it's Iron or mild steel then, not sure what they actually make it out of.
Re: Downs Cell Electrical Insulator
Ash Small, Fri Feb 06 2015, 11:19PM
I'd do a bit more research first, you need to check what happens with these elements at the proposed temperatures. I think the chlorine attacks the iron in the stainless at the grain boundaries. It causes 'pitting', so if it attacks iron in stainless, it will dissolve an iron vessel. I assume it forms ferric chloride, or something similar. The sodium might dissolve it as well.
You may need something inert, like tungsten, but, again, I'd check first.
EDIT: sodium chloride causes iron (steel) to rust (salt on the roads in winter?), molten sodium chloride will probably 'eat' it
EDIT: Phosphor bronze or monel 'may' be a better bet, they are both immune to seawater, but I'd still check first'
Ash Small, Fri Feb 06 2015, 11:19PM
I'd do a bit more research first, you need to check what happens with these elements at the proposed temperatures. I think the chlorine attacks the iron in the stainless at the grain boundaries. It causes 'pitting', so if it attacks iron in stainless, it will dissolve an iron vessel. I assume it forms ferric chloride, or something similar. The sodium might dissolve it as well.
You may need something inert, like tungsten, but, again, I'd check first.
EDIT: sodium chloride causes iron (steel) to rust (salt on the roads in winter?), molten sodium chloride will probably 'eat' it

EDIT: Phosphor bronze or monel 'may' be a better bet, they are both immune to seawater, but I'd still check first'
Re: Downs Cell Electrical Insulator
Thomas W, Fri Feb 06 2015, 11:39PM
I don't believe that chlorine or sodium would dissolve the iron/stainless steel/ mild steel.
This guy seemed to have no problem:

I think the only time chlorine causes problems with iron and stainless is when it is wet chlorine (water vapor) which won't be an issue as the pipes and the chlorine are at a high temperature.
EDIT:
Having done some more research it seem the leading method is to have a long 'neck' at the bottom of the container which is watercooled, using the solid NaCl as an insulator to protect the clay, plastic or other at the bottom.
Thomas W, Fri Feb 06 2015, 11:39PM
I don't believe that chlorine or sodium would dissolve the iron/stainless steel/ mild steel.
This guy seemed to have no problem:

I think the only time chlorine causes problems with iron and stainless is when it is wet chlorine (water vapor) which won't be an issue as the pipes and the chlorine are at a high temperature.
EDIT:
Having done some more research it seem the leading method is to have a long 'neck' at the bottom of the container which is watercooled, using the solid NaCl as an insulator to protect the clay, plastic or other at the bottom.
Re: Downs Cell Electrical Insulator
Ash Small, Sat Feb 07 2015, 12:23AM
Using NaOH (as in the link you posted) you may be ok.
You mention clay in the edit. That may be ok.
EDIT: I'd consider enamelled iron, but I'd do some research first
EDIT: If it eats glass it may eat enamel, I don't know.
EDIT: I only said the above because I've used enamelled ironware to melt aluminium as I know it works for that.
Ash Small, Sat Feb 07 2015, 12:23AM
Using NaOH (as in the link you posted) you may be ok.
You mention clay in the edit. That may be ok.
EDIT: I'd consider enamelled iron, but I'd do some research first

EDIT: If it eats glass it may eat enamel, I don't know.
EDIT: I only said the above because I've used enamelled ironware to melt aluminium as I know it works for that.
Re: Downs Cell Electrical Insulator
Thomas W, Sat Feb 07 2015, 01:18AM
I want to go with a Downs Cell specifically, The Downs Cell is an improved version of the Castner Cell that works using Sodium Chloride as opposed to Sodium Hydroxide.
Thomas W, Sat Feb 07 2015, 01:18AM
I want to go with a Downs Cell specifically, The Downs Cell is an improved version of the Castner Cell that works using Sodium Chloride as opposed to Sodium Hydroxide.
Re: Downs Cell Electrical Insulator
Ash Small, Sat Feb 07 2015, 12:41PM
Wikipedia says iron cathode, and so does this link: but this also says a vessel lined with fire bricks and an iron ring anode.
but this also says a vessel lined with fire bricks and an iron ring anode.
Ash Small, Sat Feb 07 2015, 12:41PM
Wikipedia says iron cathode, and so does this link:
 but this also says a vessel lined with fire bricks and an iron ring anode.
but this also says a vessel lined with fire bricks and an iron ring anode.Re: Downs Cell Electrical Insulator
Bored Chemist, Sat Feb 07 2015, 02:43PM
Just or the record, hot glass is an electrical conductor.
There are two ways to think about that, one is to consider it to be a problem. the other is to use it as the solution.

Bored Chemist, Sat Feb 07 2015, 02:43PM
Just or the record, hot glass is an electrical conductor.
There are two ways to think about that, one is to consider it to be a problem. the other is to use it as the solution.

Re: Downs Cell Electrical Insulator
Wolfram, Sun Feb 08 2015, 09:53PM
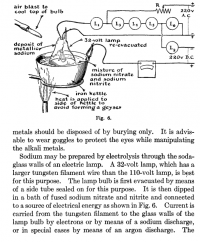
The same method is also mentioned in "Procedures in Experimental physics" by Strong from 1938. The book is out of copyright and freely available on arcive.org . This procedure is described on page 536.
. This procedure is described on page 536.
Wolfram, Sun Feb 08 2015, 09:53PM
Bored Chemist wrote ...
Just or the record, hot glass is an electrical conductor.
There are two ways to think about that, one is to consider it to be a problem. the other is to use it as the solution.

Just or the record, hot glass is an electrical conductor.
There are two ways to think about that, one is to consider it to be a problem. the other is to use it as the solution.

The same method is also mentioned in "Procedures in Experimental physics" by Strong from 1938. The book is out of copyright and freely available on arcive.org
 . This procedure is described on page 536.
. This procedure is described on page 536.
Re: Downs Cell Electrical Insulator
Ash Small, Sun Feb 08 2015, 11:21PM
There is a patent here which uses sodium carbonate and a molten lead cathode. Sodium is separated from the lead by vacuum distillation. No idea if it works, though

Ash Small, Sun Feb 08 2015, 11:21PM
There is a patent here which uses sodium carbonate and a molten lead cathode. Sodium is separated from the lead by vacuum distillation. No idea if it works, though


Re: Downs Cell Electrical Insulator
Thomas W, Sun Feb 08 2015, 11:58PM
Now you are getting complicated! haha,
I think I might put this project on the shelf for now to come back to, Get a tesla-coil built first (Fucking hell... I still haven't made one haha)
Thomas W, Sun Feb 08 2015, 11:58PM
Ash Small wrote ...
There is a patent here which uses sodium carbonate and a molten lead cathode. Sodium is separated from the lead by vacuum distillation. No idea if it works, though

There is a patent here which uses sodium carbonate and a molten lead cathode. Sodium is separated from the lead by vacuum distillation. No idea if it works, though


Now you are getting complicated! haha,
I think I might put this project on the shelf for now to come back to, Get a tesla-coil built first (Fucking hell... I still haven't made one haha)
Re: Downs Cell Electrical Insulator
Enceladus, Thu Feb 09 2017, 06:44AM
There's another way to get Na and Cl2 by electrolysis of brine instead of molten salt but it involves using a mercury anode and produces sodium amalgam rather than pure sodium in one step.
https://en.wikipedia.org/wiki/Castner%E2%80%93Kellner_process
Enceladus, Thu Feb 09 2017, 06:44AM
There's another way to get Na and Cl2 by electrolysis of brine instead of molten salt but it involves using a mercury anode and produces sodium amalgam rather than pure sodium in one step.
https://en.wikipedia.org/wiki/Castner%E2%80%93Kellner_process
Print this page